The German Federal Joint Committee (G-BA) launched the innovation fund project INTEGRATE-ATMP to standardise care structures for Advanced Therapy Medicinal Products. The ambitious project aims to provide multi-channel direct patient care communication on a national level via an innovative telemedicine platform.

Advanced therapy medicinal products (ATMPs) offer ground-breaking new ways to treat rare and severe diseases such as e.g. SMA and blood cancer.
However, these therapies require individualised care and rapid communication structures that still need to be created. The Heidelberg University Hospital-led Lighthouse Project INTEGRATE-ATMP aims to address this challenge by implementing standardised therapy plans and outpatient treatments over four years. An innovative telemedicine platform will be combined with a therapy-specific ATMP registry to monitor treatment outcomes and quality of life.
In collaboration with our partner QuickBird Medical, Designit was asked to define and develop the new platform with technological and regulatory requirements and user experience for critical care patients and HCPs operating in a highly demanding environment.
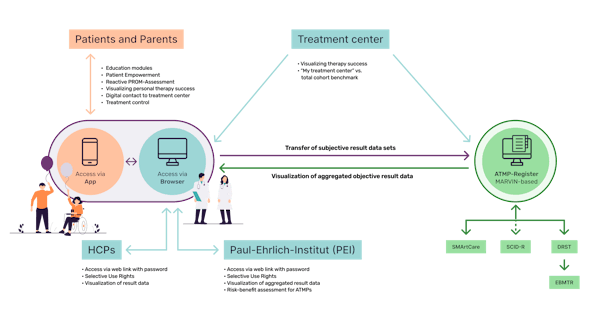
At its core, the platform introduces a ground-breaking central system that seamlessly connects previously disconnected hospital systems, registries, healthcare institutions and patients. The platform is connected to a newly established modular ATMP registry, which will serve as a new platform for future ATMP approvals.
The benefits of this connection will be better patient monitoring, early detection of medical events, better treatment adjustments and faster communication between patients and HCPs.
Creating value through user insights and participation
We actively engaged all stakeholders within the trial consortium and patients in the development process through empathy-led co-creation and participation, gaining valuable insights into their unique requirements and needs.
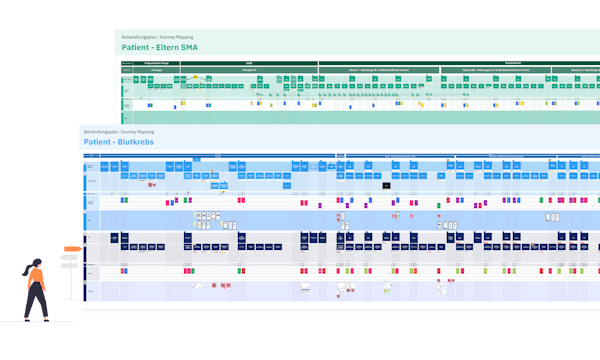
These insights were then translated into actionable recommendations, taking into account the standardised therapy plans developed in Delphi-Panels within the consortium. They have been translated into journeys for patients and HCPs. We used our proprietary frameworks like patient value Archetypes and our Health Journey Blueprints.
‘Through empathy-led participation, we build a bridge between people, institutions and technologies that creates trust and engaging experiences.’

Kerstin Michaelis
Lead Service Designer, Designit
A dual-user experience that connects patients and HCPs in a seamless and engaging way
Through an iterative and usability-driven process, we created an intuitive, easy-to-access interplay of a web dashboard for HCPs as well as a therapy companion app for the patients while considering their different user experience needs. This platform supports easy HCP-to-patient communication, e.g. organising visits, therapy tracking, health document exchange, and even support in case of side effects and emergencies.
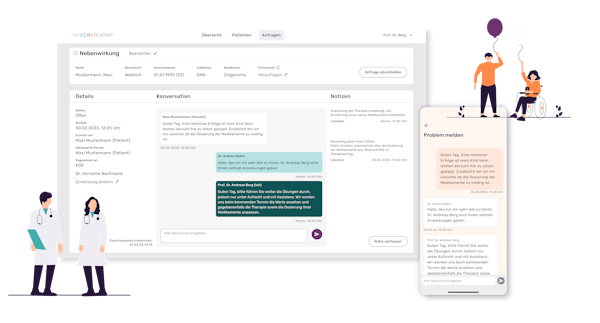
The patient app is carefully designed to organise therapy, track and measure conditions and perceived quality of life while keeping participants engaged through a play- and mindful design, preventing dropouts even if patients are in bad health conditions.


The HCP platform, instead, supports a clear and fast overview of patient data, their therapy plans and changing conditions which fluently fits into professional workflows and busy environments.
Impact
INTEGRATE-ATMP has the potential to drastically enhance personalised treatments and will act as a blueprint within the German healthcare system. Highlighting the importance of patient-centric digital healthcare solutions, the telemedicine platform adds value and fosters trust among all stakeholders by delivering the utmost in personalized information and transparency while ensuring the highest standards of personal data protection.
‘Designit's empathetic and collaborative approach ensured a seamless integration of patient, healthcare professional, and development partner perspectives into our innovative platform. Their expertise in the healthcare system, user experience, and technology solutions elevates patient satisfaction, engagement, and operational efficiency for HCPs.’

Dr. Med. Andreas Ziegler
Head of Studies, Heidelberg University Hospital
‘Designit's essential role in the platform's success underscores the value of user-centric design, making it an invaluable partner in our journey towards transformative healthcare solutions.’

Dr. med. Maria-Luisa Schubert
Head of Studies in CAR-T-cells

Members of the consortium
Charité – Universitätsmedizin Berlin, Deutsches Register für Stammzelltransplantation e. V., Friedrich-Alexander-Universität Erlangen-Nürnberg, Institut Frauengesundheit Institute Woman’s Health GmbH, Klinikum der Universität München, Techniker Krankenkasse, Technische Universität Dresden, Universitätsklinikum Erlangen, Universitätsklinikum Essen, Universitätsklinikum Frankfurt, Universitätsklinikum Hamburg-Eppendorf, Universitätsklinikum Tübingen
INTEGRATE-ATMP
Integrierte Versorgung Neuer Therapien durch Telemedizin, Empowerment, Gentherapeutika, Registeretablierung, Arzneimittelsicherheit, Therapiepfaden und Erstattung
Details
Client
Project team
Contact info for Markus Mayer
Contact info for Michael Urs
Contact info for Kerstin Michaelis
Get in touch
-

Danusch Mahmoudi
-

Markus Mayer

